Acquired Orphan Blood Disease Market to Reach US$ 17.0 Bn by 2033 | Persistence Market Research
The acquired orphan blood disease market is growing rapidly, driven by advancements in treatments, increasing chronic disease prevalence, and innovations.
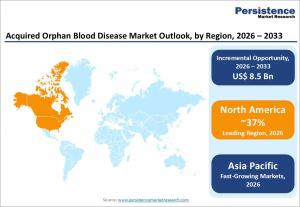
LONDON, UNITED KINGDOM, February 11, 2026 /EINPresswire.com/ -- The global acquired orphan blood disease market is expected to experience substantial growth over the next several years. With a projected value of approximately USD 8.5 billion in 2026, the market is anticipated to reach USD 17.0 billion by 2033, expanding at a compound annual growth rate (CAGR) of 10.4% from 2026 to 2033. Acquired orphan blood diseases are rare and debilitating conditions that primarily affect the blood components, including red blood cells, white blood cells, and platelets. Common disorders such as anemia, leucopenia, and thrombocytopenia result in symptoms like fatigue, weakness, infections, and bleeding.
Download Your Free Sample & Explore Key Insights: https://www.persistencemarketresearch.com/samples/4464
Market Dynamics
The growth of the acquired orphan blood disease market is primarily driven by the increasing global incidence of chronic diseases. As populations age and lifestyle-related factors such as poor diet, inactivity, and environmental factors increase, the prevalence of chronic conditions like diabetes, cancer, and autoimmune diseases has escalated. These chronic conditions often trigger complications, including rare blood disorders like paroxysmal nocturnal hemoglobinuria (PNH), aplastic anemia, and myelodysplastic syndromes (MDS), which further drives the demand for specialized treatments and therapies.
The rising burden of chronic diseases has prompted increased investments in the research and development of orphan blood disease therapies. Healthcare systems are expanding their focus on innovative treatments, advanced diagnostics, and improving patient care protocols, creating substantial growth opportunities for the acquired orphan blood disease market. However, the extended drug approval process for orphan drugs poses a significant restraint.
Orphan drug development involves lengthy clinical trials and stringent regulatory evaluations, which often delay the availability of novel treatments for patients. The longer drug approval timelines, combined with challenges in recruiting patients due to the small population size, slow down market growth.
On the other hand, advancements in genomic and molecular research are offering significant opportunities in the market. Technologies like next-generation sequencing (NGS), whole exome sequencing (WES), and CRISPR-based gene editing are revolutionizing rare blood disorder diagnosis and treatment. These advancements allow for earlier and more accurate identification of genetic variants linked to blood diseases, enabling precision medicine strategies and improving clinical outcomes.
Treatment Insights
The treatment landscape of the acquired orphan blood disease market is dominated by drug-class therapies, which are expected to hold a major share of the market, accounting for nearly 60% in 2025. This includes biologics, complement inhibitors, and immunomodulators used to treat conditions such as PNH, aplastic anemia, and MDS. Drug-based therapies are favored due to their high efficacy, safety profiles, and superior clinical outcomes, and they are expected to continue leading the market as new treatments are introduced.
Bone marrow transplantation (BMT) is the fastest-growing segment in the market. Due to its curative potential, BMT is increasingly being recognized as an effective treatment for severe blood disorders. Advances in stem cell technology, improved donor matching, and the development of reduced-intensity conditioning regimens have increased the success rates of BMT. As healthcare providers adopt BMT at an accelerating rate, particularly in emerging markets, this segment is expected to witness rapid expansion during the forecast period.
Get Custom Insights Designed for Your Business: https://www.persistencemarketresearch.com/request-customization/4464
Regional Insights
North America is expected to dominate the acquired orphan blood disease market due to its strong healthcare infrastructure, advanced diagnostic capabilities, and well-established regulatory frameworks. The U.S. leads the market, driven by extensive support for orphan drugs through initiatives like the Orphan Drug Act, which encourages the development of rare disease treatments. The market benefits from widespread availability of specialized therapies, along with a robust reimbursement system that ensures greater patient access to treatments. Innovation in the region remains strong, with several biotech firms advancing therapies like complement inhibitors and gene-editing techniques.
Meanwhile, the Asia-Pacific region is expected to emerge as the fastest-growing market. Countries such as China, Japan, and India are witnessing increasing awareness about rare hematologic diseases, along with expanding healthcare infrastructure. The adoption of advanced hematology therapies is on the rise, and more healthcare centers are offering services like bone marrow transplants. The growing presence of pharmaceutical companies in the region and the development of biosimilars are likely to further drive market growth, providing affordable access to high-cost biologics.
Competitive Landscape
These companies are heavily invested in the development of innovative therapies for orphan blood disorders. Research and strategic collaborations are key to strengthening market positions, as evidenced by Sanofi’s recent acquisition of Inhibrx to enhance its pipeline of biologic candidates.Other notable players in the market include GlaxoSmithKline, Cyclacel Pharmaceuticals, and Onconova Therapeutics. These companies are continually innovating and diversifying their product offerings, contributing to the overall growth and development of the market.
Checkout Now & Download Complete Market Report: https://www.persistencemarketresearch.com/checkout/4464
Market Segmentation
By Treatment
Drug Class
Antineoplastic drugs
Demethylation Agents
Immunosuppressants
Immunomodulators
Monoclonal Antibodies
Others
Transfusion or Exchange
Bone Marrow Transplant
By Disease Type
Acquired thrombotic thrombocytopenic purpura (aTTP)
Cold Agglutinin Disease (CAD
Paroxysmal Nocturnal Hemoglobinuria (PNH)
Polycythemia Vera
Myelodysplastic Syndrome (MDS)
Others
By Distribution Channel
Hospital Pharmacy
Retail Pharmacy
Online Pharmacies
Others
By Region
North America
Europe
East Asia
South Asia and Oceania
Latin America
Middle East and Africa
Read Related Reports:
Molecular Diagnostics Reagent Market: The molecular diagnostics reagent market will reach US$15.8 Bn by 2032, driven by rising disease testing, personalized medicine, and PCR advancements
Healthcare Quality and Safety Reporting System Market: The global healthcare quality and safety reporting market is valued at US$1.4 Bn in 2025 and projected to reach US$3.0 Bn by 2032, growing at a 12% CAGR.
Persistence Market Research
Persistence Market Research Pvt Ltd
+1 646-878-6329
email us here
Visit us on social media:
LinkedIn
Instagram
Facebook
YouTube
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.